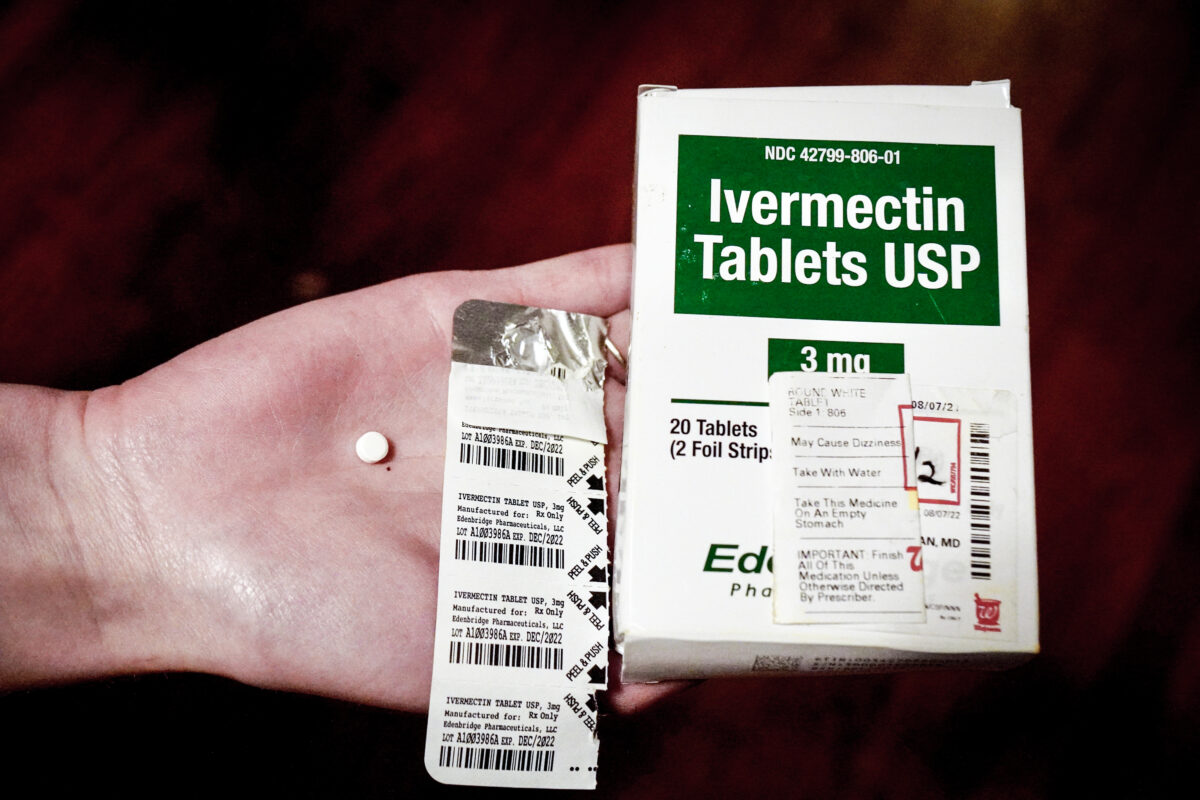
Oklahoma’s attorney general has told doctors across the state that they can prescribe ivermectin or hydroxychloroquine for the purpose of treating COVID-19 and will not face disciplinary procedures for doing so.
In a statement published on Tuesday, Attorney General John O’Connor said he has found “no legal basis” for a state medical licensure board to discipline a licensed physician for “exercising sound judgment” and “safely prescribing” the drugs.
Neither ivermectin nor hydroxychloroquine has been approved for use in treating COVID-19 by the U.S. Food and Drug Administration (FDA) but O’Connor made clear that doctors would not face penalties if they prescribed either drug for the off-label purpose of treating a patient with COVID-19.
“I stand behind doctors who believe it is in their patients’ best interests to receive ivermectin and hydroxychloroquine,” said O’Connor. “Our healthcare professionals should have every tool available to combat COVID-19. Public safety demands this. Physicians who prescribe medications and follow the law should not fear disciplinary action for prescribing such drugs.”
The attorney general’s office added that it “maintains that proper healthcare decisions are to be made between a patient and his or her physician, and the government should not interfere with their relationship.”
Both ivermectin and hydroxychloroquine have been highly controversial and dividing drugs throughout the pandemic.
Ivermectin, an anti-parasite drug, has been used by the World Health Organization for over 30 years to treat certain infections as well as head lice and skin conditions. The FDA has not approved it for use in the treatment of COVID-19 and warns that current data does not show it is effective against the virus.
Taking large doses of ivermectin can be dangerous.
However, clinical trials assessing the drug for the prevention or treatment of COVID-19 in people are ongoing, the FDA says.
Despite the warning from U.S. health authorities, all or part of 22 countries across the globe have approved the use of ivermectin in the treatment of COVID-19, based on multiple studies.
Japan has not yet approved it for the treatment of COVID-19 but a Japanese conglomerate last month found, based on lab-based research, that ivermectin had an antiviral effect on the Omicron variant of the virus in vitro studies.
The pharma firm is also currently carrying out a Phase 3 clinical trial of the drug to see if it is effective in treating COVID-19 patients.
The company said that ivermectin has the “same antiviral effect” on all “mutant strains,” including Alpha, Delta, and Omicron, and worked by suppressing the invasion of the virus and inhibiting its replication.
“[Ivermectin] is expected to be applied as a therapeutic drug (tablet) for all new coronavirus infectious diseases,” the report said.
Earlier this month, lawmakers in Iowa’s state Legislature advanced a measure that would allow ivermectin to be used to treat critically sick COVID-19 patients who are on ventilators.
The FDA also cautions people against using the anti-malaria drug hydroxychloroquine or chloroquine as a COVID-19 treatment outside of a hospital setting due to the risks of increased heart rhythm problems and other safety issues.
Hydroxychloroquine gained prominence but has also been heavily scrutinized after former President Donald Trump said he was taking it as a prophylactic.
A study published in the American Journal of Medicine on Jan. 1 found that hydroxychloroquine helped lower mortality in the early treatment of COVID-19.
However, both the FDA and World Health Organization (WHO) have advised against the use of hydroxychloroquine for COVID-19.